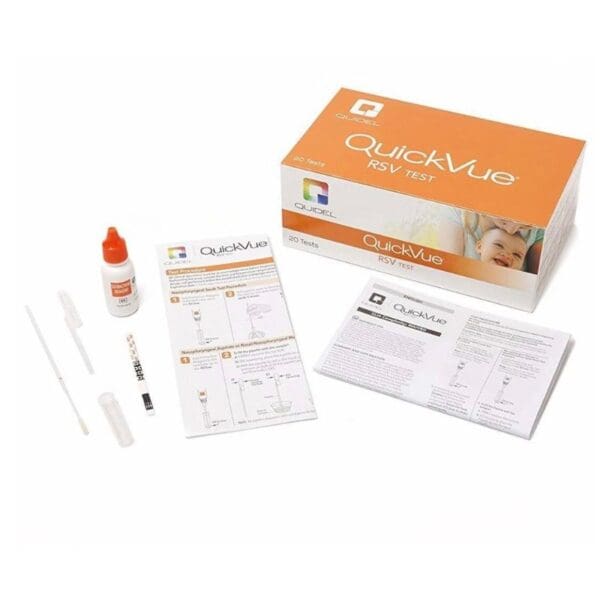
QuickVue RSV Test
QuickVue Respiratory Test Kit Expiration Date: March, 2025
Results in 15 minutes
The QuickVue RSV Test is a rapid diagnostic tool that can detect respiratory syncytial virus (RSV) from nasopharyngeal swabs, nasopharyngeal aspirates, or nasal/nasopharyngeal wash specimens from symptomatic pediatric patients.
FDA Cleared and CLIA Waived. (FDA 510K#: K061008)
What is inside the box of the QuickVue RSV Test Kit?
- (20) Individually Packaged Test Strips
- (1) Extraction Reagent Bottle With Detergents and 0.2% Sodium Azide
- (20) Extraction Tubes
- (20) Disposable Droppers
- (20) Nasopharyngeal Swabs
- (1) Positive RSV Control Swab Coated With Noninfectious RSV Antigen
- (1) Negative Control Swab Coated With Formalin-Inactivated, Noninfectious Streptococcus C Antigen
- (1) Package Insert
- (1) Quick Reference Instructions
QuickVue RSV Product Features
- Easy-To-Read Two-Color Results
- Easy-To-Use Dipstick
- Fewer Steps With Only One Reagent
- One Office Visit To Test and Treat
- Controls To Verify Test Strip Integrity
- Includes All Components Needed
- Stored at Room Temperature
Intended Use
The test is intended for use as an aid in the diagnosis of acute respiratory syncytial viral infections. It is recommended that negative test results be confirmed by cell culture. Negative results do not preclude RSV infection and it is recommended that they not be used as the sole basis for treatment or other management decisions.
Interpretation of Results See
Quick Reference Instructions for larger images of test results in COLOR. POSITIVE Result*: At 15 minutes, the appearance of ANY shade of a pink-to-red Test Line AND a blue procedural Control Line indicates a positive result for the presence of RSV viral antigen. Results will remain stable for 5 minutes after the recommended read time. *A positive result does not rule out co-infections with other pathogens.
C = Control Line
T = Test Line
*Look closely! If you see a very faint, pink Test Line and a blue Control Line, you must report the result as POSITIVE.Shipping and Returns Terms and Conditions
How Long Does It Take For My Item(s) To Ship?
Items are shipped Monday – Friday from our warehouse. 1-2 Day Processing Time.
Delivery times vary according to your selected delivery address, the availability of your items, and the time of day you place your order.
Orders placed by 1:00 PM Eastern Time will be shipped the same day from our warehouse. Orders placed after this time will be shipped the next business day.
For B2B accounts, please get in touch with us at
[email protected] for urgent order requests.
Please feel free to visit our
Support Page for FDA Extension Letters, Submit a Ticket, and more.
What Shipping Methods Do You Offer?
At Peach Medical Corp we use UPS (most orders ship via UPS but some may ship via USPS) as our logistic and Shipping partners. Currently, our shipping methods are as follows:
- UPS Next Day Air
- UPS 2nd Day Air
- UPS Ground (3-5 Day Delivery)
When Will I Receive My Tracking Number?
Our system updates clients every evening with tracking numbers for their orders. This will be sent via e-mail with an additional copy of your receipt.
Incorrect Address
Please verify your address when ordering, we are unable to fix an incorrect shipping address on an order once it is in transit. If your order is in transit with an incorrect address, please reach out to your carrier to reroute the package. Peach Medical Corp. is not able to have packages rerouted or contact the carrier for you.
Refunds will not be issued for packages that were shipped to an incorrect address but it may be possible for a replacement package to be sent, please contact the customer service team to see if you are eligible.
Return Policy and Cancellation Policy
The Peach Medical COVID-19 Antigen Rapid Test and all face masks are Non-returnable; Non-refundable as they are considered personal-use items. Replacement may be shipped if the item is defective. Proof of purchase, photos of defects, lot numbers, and other information are required to authorize a replacement. Please reach out to
customer service for detailed requirements before disposing of the defective units. We will not replace your COVID-19 Antigen Rapid Test kits that are expiring soon based on the printed label. Our expiration dates have been shown on each product page. More information can be found at
support.peachmedical.com/portal/en/kb/peachmedicalsourcing. We will continue to update the FDA documents for shelf-life extensions for our tests.
If a customer chooses a 3rd party receiver who is not the purchaser, Peach Medical is not responsible for any goods once they leave our warehouse and when tracking is provided. I.e Gifts, International Shipping Services. All Products must be packed in the original, unopened, and unmarked packaging including any accessories, manuals, documentation, and registration that shipped with the Product.
Note: If Peach Medical has not shipped your item within 7 business days, cancellations will be honored. Please email us at
[email protected] for return authorization and the shipping address.
Frequently Asked Questions For Quidel QuickVue RSV Test Kit
What is the CLIA complexity of the QuickVue RSV test?
The test is CLIA waived.
Who may be tested with the Quidel QuickVue RSV kit?
The test is designed for symptomatic pediatric patients 18 years of age and younger.
What is the shelf life of the QuickVue kit?
The shelf life is 24 months from the date of manufacture. The kit should be stored at room temperature 15°C to 30°C.
How accurate is the QuickVue RSV Test?
Nasopharyngeal aspirate sensitivity is 99% and specificity is 92%. Nasopharyngeal swab sensitivity is 92% and specificity is 92%. Nasal/nasopharyngeal wash sensitivity is 83% and specificity is 90%. These data were obtained in comparison to culture.
What is RSV?
Respiratory syncytial (sin-SISH-uhl) virus, or RSV, is a common respiratory virus that usually causes mild, cold-like symptoms. Most people recover in a week or two, but RSV can be serious, especially for infants and older adults. RSV is the most common cause of bronchiolitis (inflammation of the small airways in the lung) and pneumonia (infection of the lungs) in children younger than 1 year of age in the United States.










Reviews
There are no reviews yet.