QuickVue At-Home OTC COVID-19 Test Kit, 2 CT
QuickVue By Quidel Expiration Date: January 2025 (Rapid Results In 10 Minutes)
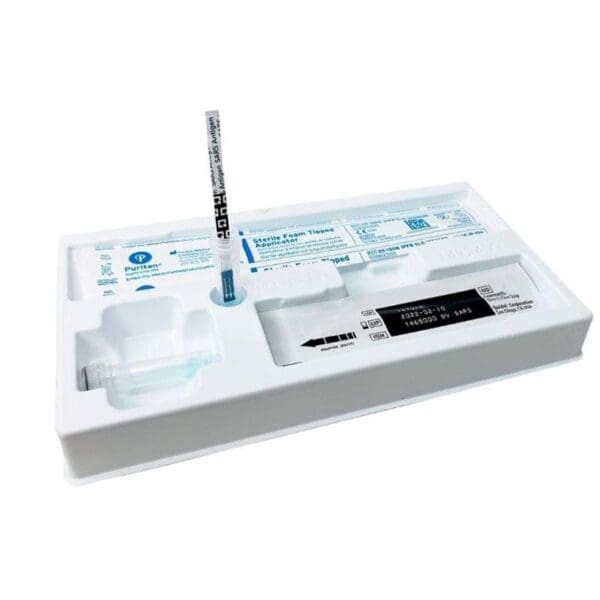
The QuickVue At-Home OTC COVID-19 Test by Quidel Corporation is a convenient and easy-to-use test that allows individuals to test for COVID-19 from the comfort of their own homes. This rapid test works by detecting the presence of the nucleocapsid protein antigen from the SARS-CoV-2 virus, which is the virus responsible for causing COVID-19.
The test is designed as a lateral flow assay, which means that a sample (such as a nasal swab) is applied to the test device and then migrates along the surface of the test where it interacts with specific antibodies that are designed to detect the presence of the viral antigen.
The QuickVue At-Home OTC COVID-19 Test is intended for qualitative detection, meaning that it can determine whether the antigen is present or absent in the sample. It provides results quickly, usually within 10-15 minutes, allowing individuals to know their COVID-19 status without having to wait for a lab to process their sample.
Overall, the QuickVue At-Home OTC COVID-19 Test is a valuable tool for individuals who want to quickly and easily test for COVID-19 in the comfort of their own home.
Peach Medical is is an authorized distributor of Quidel products. Purchase Quickvue Self Test Online with confidence.
Quickvue Covid Test Accuracy
In a clinical study, the QuickVue covid test identified positive cases 83.5% of the time, and identified negative cases 99.2% of the time (83.5% PPA, and 99.2% NPA, respectively), when compared to the PCR Test. For more information on comparing Antigen Tests and PCR test, please check out our recent article.
- Results: 10 Minutes
- Specificity: 83.5%
- Sensitivity: 99.2%
Quickvue At-home COVID Test Instructions
The Quickvue At-Home OTC COVID-19 test can accurately detect the Omicron variant (among others) of the Sars-CoV-2, the potentially deadly virus that can lead to COVID-19.
Quickvue at home COVID Test Instructions
QuickVue At-Home OTC COVID-19 Product Documents
The QuickVue At-Home OTC COVID-19 Test is intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 from individuals within 6-days of symptom onset or individuals without symptoms or other epidemiological reasons to suspect COVID-19 infection when tested twice over three days with at least 24 hours (and no more than 48 hours) between tests.
For the most up-to-date information on COVID-19, please
visit the CDC’s Website
DISCLAIMER
Please note that all Peach Medical shipments do not require a signature upon delivery. Since most test kits should be stored between 35.6 and 86 degrees F range, we strongly recommend tracking your order and anticipating its delivery. However, if the test is stored outside the temperature range for a relatively short period – for a couple of hours up to a day or two – it will be fine to use as long as the test and its components are used at room temperature. Peach Medical is not responsible if the test kits are left outside in extreme temperatures for longer than the recommended time frame after delivery.
The QuickVue At-Home OTC COVID-19 Test is a lateral flow immunoassay device intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2.
The QuickVue At-Home OTC COVID-19 Test authorized for non-prescription home use with self-collected direct anterior nasal (nares) swab samples from individuals aged 14 years or older or adult-collected anterior nasal (nares) swab samples from individuals aged two years or older. This test is authorized for individuals with symptoms of COVID-19 within the first six days of symptom onset when tested at least twice over three days with at least 48 hours between tests, and for individuals without symptoms or other epidemiological reasons to suspect COVID-19, when tested at least three times over five days with at least 48 hours between tests. The QuickVue At-Home OTC COVID-19 Test does not differentiate between SARS-CoV and SARS-C0V-2.
In the USA, the Quidel Quickvue Covid Test has not been FDA cleared or approved but has been authorized by FDA under an Emergency Use Authorization. This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
Shipping and Returns Terms and Conditions
How Long Does It Take For My Item(s) To Ship?
Items are shipped Monday – Friday from our warehouse. 1-2 Day Processing Time.
Delivery times vary according to your selected delivery address, the availability of your items, and the time of day you place your order.
Orders placed by 1:00 PM Eastern Time will be shipped the same day from our warehouse. Orders placed after this time will be shipped the next business day.
For B2B accounts, please get in touch with us at
[email protected] for urgent order requests.
Please feel free to visit our
Support Page for FDA Extension Letters, Submit a Ticket, and more.
What Shipping Methods Do You Offer?
At Peach Medical Corp we use UPS (most orders ship via UPS but some may ship via USPS) as our logistic and Shipping partners. Currently, our shipping methods are as follows:
- UPS Next Day Air
- UPS 2nd Day Air
- UPS Ground (3-5 Day Delivery)
When Will I Receive My Tracking Number?
Our system updates clients every evening with tracking numbers for their orders. This will be sent via e-mail with an additional copy of your receipt.
Incorrect Address
Please verify your address when ordering, we are unable to fix an incorrect shipping address on an order once it is in transit. If your order is in transit with an incorrect address, please reach out to your carrier to reroute the package. Peach Medical Corp. is not able to have packages rerouted or contact the carrier for you.
Refunds will not be issued for packages that were shipped to an incorrect address but it may be possible for a replacement package to be sent, please contact the customer service team to see if you are eligible.
Return Policy and Cancellation Policy
The Peach Medical COVID-19 Antigen Rapid Test and all face masks are Non-returnable; Non-refundable as they are considered personal-use items. Replacement may be shipped if the item is defective. Proof of purchase, photos of defects, lot numbers, and other information are required to authorize a replacement. Please reach out to
customer service for detailed requirements before disposing of the defective units. We will not replace your COVID-19 Antigen Rapid Test kits that are expiring soon based on the printed label. Our expiration dates have been shown on each product page. More information can be found at
support.peachmedical.com/portal/en/kb/peachmedicalsourcing. We will continue to update the FDA documents for shelf-life extensions for our tests.
If a customer chooses a 3rd party receiver who is not the purchaser, Peach Medical is not responsible for any goods once they leave our warehouse and when tracking is provided. I.e Gifts, International Shipping Services. All Products must be packed in the original, unopened, and unmarked packaging including any accessories, manuals, documentation, and registration that shipped with the Product.
Note: If Peach Medical has not shipped your item within 7 business days, cancellations will be honored. Please email us at
[email protected] for return authorization and the shipping address.
Frequently Asked Questions For QuickVue At-Home Antigen COVID-19 Test Kit
How to use QuickVue COVID test?
Carefully insert the swab ½ to ¾ of an inch into the nostril, adjusting based on the person’s nose size. Firmly rotate the swab in a circular motion around the inside wall of each nostril at least 4 times. Make sure to use the same swab for both nostrils. Note: If you are swabbing others, please wear a face mask.
Are QuickVue At-Home tests accurate?
Clinical studies have shown that antigen tests more accurately determine whether you are infected with the virus that causes COVID-19 when taken multiple times across several days. Repeat testing improves test accuracy. This serial testing approach is recommended to minimize the risk of incorrect results. For more information on the performance of the test, please refer to the performance data in the Healthcare Provider Instructions for Use (IFU).
How long does it take to get results from the QuickVue COVID-19 test kit?
Results are available in as little as 10 minutes in the privacy of your own home.
How many tests come in a QuickVue At-Home kit?
The test kit comes with two tests intended to be used for the same patient. A 25-test kit is also available.
Is a prescription required to perform this QuickVue At-Home Antigen COVID-19 Test Kit?
You do not need a doctor’s prescription to purchase and perform this test.
What is the difference between an antigen test and PCR or molecular tests?
There are different kinds of tests for the SARS-CoV-2 virus that causes COVID-19. Molecular tests detect genetic material from the virus. Antigen tests, such as the QuickVue COVID-19 Test, detect proteins from the virus.
What is the age range for the QuickVue at-home test?
This test is authorized for nonprescription home use with self-collected (unobserved) direct anterior nasal (NS) swab specimens from individuals aged 14 years and older, or with adult-collected anterior NS samples from individuals aged 2 years or older.













Tania P –
Ordered this test kit for my family gathering. I found it easy to use. The directions are clear and the results are easy to read.
Jessica Ciampa –
Fast service as always! Thank you
Davi –
These at home tests are lifesavers. Easy to use and got my results in 10 mins. Fast shipping too!
Brian P. –
I got this “at home” test from Quidel. I needed to be able to test at home for possible work exposure reasons. I was a little skeptical since every other test seems to try to pierce my frontal lobe like a “wanna be labotomy” spear! This however just went a little bit into my nose and a couple swirls later…. I was done! Couldn’t believe the simplicity. I swear I heard my brain jamming to Bob Marley’s “Everything little thing gonna be alright!” A few minutes later after using the “instructions for dummies” guide….. results were read! Neg-a-tive good buddy! So let this be proof that it’s so easy….. “Even a Caveman like me can do it!”
Nitin K. –
o we do keep a few of these in the house. The family is vaccinated at this point however with all these variants flying around we feel it’s important to have them in the house. Summer colds are a thing too- so this may help rule out Covid in those instances.
Be cautioned- this is in now way a substitute for a vaccine. Vaccines save life’s.
These tests do have a margin or error for false negative and false positive. You should not rely soley on this tests response as a way to gauge how you interact and where you go around people. It should be taken with a grain of salt and if something doesn’t seem right (I.E: you feel sick with Covid symptoms and this still reports negative) you should probably get a professionally administered Covid test because there is a margin of error with these tests, which is why it comes with 2 to use – but even then it may be incorrect.
Negative tests are not entirely enough additionally- these tests are not always accepted as the gold standard for activities such as international travel and work related needs.
Vaccines save lives.
Hillary –
Over the last weekend found out I was in contact with someone earlier in the week who had Covid, I just couldn’t wait the 2-3 days for a PCR test so I got this. Very easy to use, took both tests 36 hours apart – both negative!
Maynard –
Had a bad cold but wanted to make sure it wasn't covid. Used this product and followed directions exactly. Tested negative. Went to a clinic next day and tested negative there as well. I'd recommend this product.
Debra –
Pretty easy to use instructional video for adults and children. We used this test for traveling during the holidays to our parents house during the thanksgiving holidays. I would recommend to use the test kit with gloves.
Adammalix –
We needed a few hundred of these for our workplace. After searching high and low we finally found the right provider! Thank you so much peach medical for making this so easy!
Nancy –
We could not find these tests anywhere but needed them for our nursery to reopen. Easy to use.