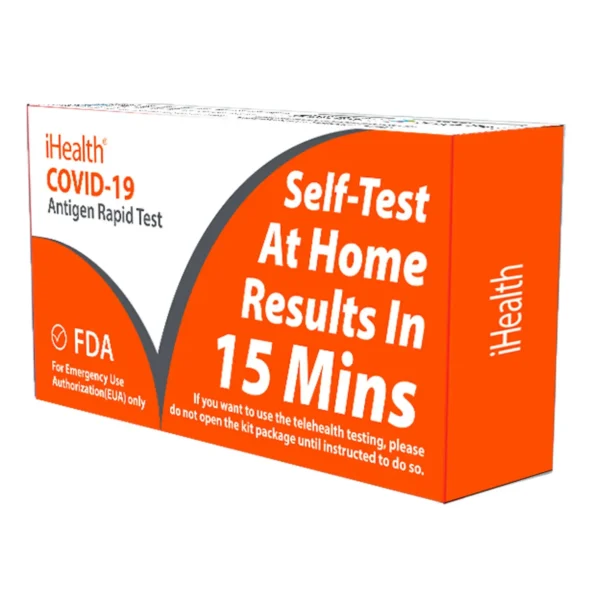
iHealth COVID-19 Test Kit
IHealth Covid Test Expiration Date: January 5th, 2025
The iHealth Covid tests are a 15-minute self-test to detect nucleocapsid protein antigen from SARS-CoV-2. The test can be completed in the comfort of your own home without the need to ship your sample to a lab.
IHealth Covid 19 Antigen Test Accuracy
- Nasal Swab: 98.8% (95% CI: 97.6%-99.5%) 95% Confidence Intervals
- Saliva: 97.1% (95% CI: 94.6%-98.5%) 95% Confidence Intervals
What Is In The iHealth Covid Test Antigen Home Test Box?
- 2 Test devices: Foil pouched test device containing one test strip which is encased in plastic device cassette with a desiccant
- 2 Extraction vials and caps
- 2 Nasal swabs: swab for anterior nasal specimen collection
The iHealth covid test kit contain two tests per box and is authorized for non-prescription home use. iHealth Covid tests are perfect for organizations and facilities that need to test staff or who need to make COVID-19 test kits available to check for potential exposures.
How to Use the iHealth Covid 19 Antigen Rapid Test
Each iHealth Covid Test Kit contains instructions. These instructions can also be downloaded from the iHealth COVID-19 Antigen Rapid Test app:
- Open the kit and remove all items from the test kit.
- Remove the swab from its package and place the entire tip of the swab, up to ¾ of an inch, into one nostril.
- Carefully move the swab in circles at least five times, brushing the insides of the nostril.
- Repeat with the other nostril using the same swab.
- Take the tube, tap it on a table and open the orange cap.
- Place the swab fully into the tube until it touches the bottom, and swirl it in the tube at least 15 times.
- Squeeze the sides of the tube to squeeze out more of the sample from the swab and then throw away the swab.
- Put the cap back on the tube and open the small white cap.
- Squeeze three drops from the tube into the sample well of the test card.
- Wait fifteen minutes to read the results.
EASY TO USE ZERO DISCOMFORT
The test can be done by inserting only 1/2 to 3/4 inches with a simple non-invasive nasal swab, easy to use, and has zero discomfort. Step-by-step instructional videos are available in our app for easy following. (Installation of the app is optional.)
DETECT CURRENT AND NEW COVID VARIANTS
iHealth has completed in-lab testing on several heat-inactivated variant strains and the results show that the iHealth COVID-19 Antigen Rapid Test is able to detect the mutations of both the Delta and Omicron variants.
GROUP TESTING MANAGING VIA MOBILE APP
The iHealth COVID-19 Test app allows the administrator of a small group to monitor and track the group members’ test results when needed at school, work, or events.
The iHealth® COVID-19 Antigen Rapid Test is a lateral flow assay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2.
The iHealth Covid Test is authorized for non-prescription home use with self-collected anterior nasal (nares) swab samples from individuals aged 15 years or older with symptoms of COVID-19 within the first 7 days of symptom onset. The iHealth Covid test is authorized for non-prescription home use with adult-collected nasal swab samples from individuals aged 2 years or older with symptoms of COVID-19 within the first 7 days of symptom onset.
Buy Ihealth Covid 19 Test Online With Peach Medical
Peach Medical is the leader in diagnostic testing equipment for both clinical and home use. With over 100,000+ purchases online, choose the best vendor for your organizations needs.
Important Documents
Please call us at 1-877-597-3224 or e-mail us at [email protected] for wholesale pricing. We are more than happy to provide references within production, government, finance, education, fortune 500 companies, and more. Over 1,000,000+ orders of test kits sold over the past year.
DISCLAIMER
Peach Medical shipments do not require a signature upon delivery to your door. Most Antigen test kits should be stored between 35.6 and 86 degrees. Peach Medical recommends that you track your order and anticipate its delivery. However, if the tests are stored outside for a relatively short time. Ie. for a couple of hours up to a day or two – it should he fine to use as long as the test and its components are used at room temperature.
Please Note: Peach Medical is not responsible if the test kits are left outside in extreme temperatures for longer than the recommended time frame after delivery.
EUA Info from the FDA:
- The iHealth™ COVID-19 Antigen Home Test is authorized for non-prescription home use with self-collected direct anterior nasal (nares) swab samples from individuals aged 14 years or older or adult collected direct anterior nasal swab samples from individuals aged two years or older.
- For use under an Emergency Use Authorization (EUA) only for the duration of the COVID-19 declaration justifying the emergency use of in vitro diagnostics (IVD), unless it is terminated or revoked by the FDA (after which the test may no longer be used).
Shipping and Returns Terms and Conditions
How Long Does It Take For My Item(s) To Ship?
Items are shipped Monday – Friday from our warehouse. 1-2 Day Processing Time.
Delivery times vary according to your selected delivery address, the availability of your items, and the time of day you place your order.
Orders placed by 1:00 PM Eastern Time will be shipped the same day from our warehouse. Orders placed after this time will be shipped the next business day.
For B2B accounts, please get in touch with us at
[email protected] for urgent order requests.
Please feel free to visit our
Support Page for FDA Extension Letters, Submit a Ticket, and more.
What Shipping Methods Do You Offer?
At Peach Medical Corp we use UPS (most orders ship via UPS but some may ship via USPS) as our logistic and Shipping partners. Currently, our shipping methods are as follows:
- UPS Next Day Air
- UPS 2nd Day Air
- UPS Ground (3-5 Day Delivery)
When Will I Receive My Tracking Number?
Our system updates clients every evening with tracking numbers for their orders. This will be sent via e-mail with an additional copy of your receipt.
Incorrect Address
Please verify your address when ordering, we are unable to fix an incorrect shipping address on an order once it is in transit. If your order is in transit with an incorrect address, please reach out to your carrier to reroute the package. Peach Medical Corp. is not able to have packages rerouted or contact the carrier for you.
Refunds will not be issued for packages that were shipped to an incorrect address but it may be possible for a replacement package to be sent, please contact the customer service team to see if you are eligible.
Return Policy and Cancellation Policy
The Peach Medical COVID-19 Antigen Rapid Test and all face masks are Non-returnable; Non-refundable as they are considered personal-use items. Replacement may be shipped if the item is defective. Proof of purchase, photos of defects, lot numbers, and other information are required to authorize a replacement. Please reach out to
customer service for detailed requirements before disposing of the defective units. We will not replace your COVID-19 Antigen Rapid Test kits that are expiring soon based on the printed label. Our expiration dates have been shown on each product page. More information can be found at
support.peachmedical.com/portal/en/kb/peachmedicalsourcing. We will continue to update the FDA documents for shelf-life extensions for our tests.
If a customer chooses a 3rd party receiver who is not the purchaser, Peach Medical is not responsible for any goods once they leave our warehouse and when tracking is provided. I.e Gifts, International Shipping Services. All Products must be packed in the original, unopened, and unmarked packaging including any accessories, manuals, documentation, and registration that shipped with the Product.
Note: If Peach Medical has not shipped your item within 7 business days, cancellations will be honored. Please email us at
[email protected] for return authorization and the shipping address.
IHealth COVID-19 Rapid Test Frequently Asked Questions
How does the IHealth COVID-19 Antigen Rapid Test work?
The iHealth® COVID-19 Antigen Rapid Test is an antigen test. When COVID-19 is present in the body, your nasal secretions can also contain the SARS-CoV-2 virus (the virus that causes COVID-19). The iHealth® COVID-19 Antigen Rapid Test can detect small parts of the SARS-CoV-2 virus, known as N protein or antigens, in your nasal secretions.
Will this test detect COVID-19 variants?
The iHealth COVID test has completed testing on several heat-inactivated variant strains and the iHealth® COVID-19 Antigen Rapid Test was able to detect the mutations. The clinical performance has not been established in all circulating variants but is anticipated to be reflective of the prevalent variants in circulation at the time and location of the clinical evaluation. Performance at the time of testing may vary depending on the variants circulating, including newly emerging strains of SARS-CoV-2 and their prevalence, which change over time.
Is this iHealth COVID-19 Antigen Rapid Test Kit FDA approved or cleared?
No. This test is not yet approved or cleared by the United States FDA. FDA can issue an Emergency Use Authorization (EUA) when certain criteria are met, which includes that there are no adequate, approved, available alternatives. The EUA for this test is supported by the Secretary of Health and Human Services’ (HHS’s) declaration that circumstances exist to justify emergency use of in vitro diagnostics for the detection and/or diagnosis of the virus that causes COVID-19. This EUA will remain in effect (meaning this test can be used) for the duration of the COVID-19 declaration justifying emergency of IVDs, unless it is terminated or authorization is revoked by FDA (after which the test may no longer be used).
What is the age range for the test?
This test is authorized for nonprescription home use with self-collected (unobserved) anterior nasal swab specimens from individuals aged 15 years and older or with adult-collected anterior nasal swab samples from individuals aged 2 years or older.
What is serial testing?
Serial testing is when a single person is tested for COVID-19 more than once. Because antigen tests are less sensitive than other COVID-19 tests and false results may occur, repeated testing may identify individuals with COVID-19 more reliably than a single test. By repeating testing, it may be possible to more quickly identify cases of COVID-19 and reduce spread of infection. Additional testing with molecular COVID-19 tests may be necessary, depending on your individual risk factors and test results. It is important that you work with your healthcare provider to help you understand the next steps you should take. Serial testing (i.e., testing every day or every other day) is more likely to detect COVID-19, especially when you do not have any symptoms. Testing for asymptomatic individuals should be performed at least twice over three days, with at least twenty-four hours and no more than 48 hours between tests. You may need to purchase additional tests to perform this serial (repeat) testing.
Do I always need to perform two tests?
If you have COVID-19 symptoms and are within the first 7 days of symptom onset, you can use one single test of the iHealth® COVID-19 Antigen Rapid Test. You may also choose to perform two tests if you wish. By testing more frequently, you may detect COVID-19 more quickly and reduce spread of infection. If you do not have COVID-19 symptoms or have been having symptoms for more than 7 days, you will need to perform two tests over two or three days with at least 24 hours and no more than 48 hours between tests.
How accurate is the iHealth COVID-19 Antigen Rapid Test?
Based on the results of a clinical study where the iHealth® COVID-19 Antigen Rapid Test was compared to an FDA authorized molecular SARS-CoV-2 test, the iHealth® COVID-19 Antigen Rapid Test correctly identified 94.3% of positive specimens and 98.1% of negative specimens. Additional asymptomatic individuals and individuals beyond the seven days of symptom onset were tested, but excluded from the primary performance calculations because they were not included in the intended use. A higher proportion of low positive specimens were observed in these populations, resulting in PPAs between 85-88% in these individuals.













iHealth COVID-19 Self-Test At-Home. –
Great customer service
Diane Drobia –
Great customer service
iHealth COVID-19 Self-Test At-Home. –
There was a storm delay that delayed shipping. They notified us as soon as it occurred so we were aware. The Test Kits were exactly as described and were actually delivered pretty quick
Gregory L Shoop –
There was a storm delay that delayed shipping. They notified us as soon as it occurred so we were aware. The Test Kits were exactly as described and were actually delivered pretty quick
iHealth COVID-19 Self-Test At-Home. –
just what we needed, simple and safe…
Juan Pablo Conde –
just what we needed, simple and safe…
iHealth COVID-19 Self-Test At-Home. –
Fast delivery
Erica Grant –
Fast delivery
iHealth COVID-19 Self-Test At-Home. –
Easy To Use.
Tayyab –
Easy To Use.
iHealth COVID-19 Self-Test At-Home. –
I have used these test multiple times during this crazy pandemic. Reliable, trustworthy. My only complaint this time is one of vials is missing the fluid. I bought a 5 pack test. But in essence, I only got 4 test. It would be nice to get a replacement for this test, but I don’t have time for that! My suggestion to company- make sure your QC is on point. Please.
Mr.B –
I have used these test multiple times during this crazy pandemic. Reliable, trustworthy. My only complaint this time is one of vials is missing the fluid. I bought a 5 pack test. But in essence, I only got 4 test. It would be nice to get a replacement for this test, but I don’t have time for that! My suggestion to company- make sure your QC is on point. Please.
iHealth COVID-19 Self-Test At-Home. –
No comment.
iHealth COVID-19 Self-Test At-Home. –
Unable to find tests in stores. Arrived quickly! Will buy from Peach again, thanks.
Carolyn Vega –
Unable to find tests in stores. Arrived quickly! Will buy from Peach again, thanks.
iHealth COVID-19 Self-Test At-Home. –
Super accurate test and they delivered so quick. I ordered the night before and got it next afternoon.
Mark T. –
Spoke to Danielle, she was very helpful. My package arrived on time and I'm very happy to have these tests. Never know when you need them.
Jessie A. –
These tests were extremely helpful to have during Omicron. I was waiting for my test from the Gov but decided I needed some in the house just in case. Very good decision. They shipped quickly. Like the small packaging. Tests on the go!
iHealth COVID-19 Self-Test At-Home. –
Nitin assisted me when getting this product for my contractors. The test is amazing and easy to use. The delivery was on time just as nitin indicated. I will keep buying more for sure!
Mia S. –
I’m in the restaurant industry and needed a few kits to make sure all of our employees were negative before working. I got the kits on time and they were great. The directions were easy to understand and shown to be very reliable. Will definitely order more kits from this supplier.
Ana –
Nitin assisted me when getting this product for my contractors. The test is amazing and easy to use. The delivery was on time just as nitin indicated. I will keep buying more for sure!
DIGSMA –
Ordered the iHealth box of 6. Single packs were sold out. Got an email they won’t be here till 1/6. My flu symptoms & my daughter’s have subsided & had to quarantine entire time, not knowing if we have A flu or The flu. Son & husband have no symptoms. Getting a corona appt or at home test is like getting Biden to provide those ‘free’ at home tests he speaks of that don’t exist. This is ludicrous. People with symptoms can’t get a test. People without any symptoms hoarding tests, like toilet paper. I can’t. By the time I get the 6 I ordered, I’ll probably have a new strain of something these tests won’t detect anyway. Wish they came quicker. But not peach’s fault… “Administration didn’t see it coming” Also noticed peach’s tests went up in price significantly since 12/28 when I ordered… supply and demand.
iHealth COVID-19 Self-Test At-Home. –
We tried the test before visiting our parents for the holidays. Easy to use, small packaging. The video online really helped.
Nathan S. –
We tried the test before visiting our parents for the holidays. Easy to use, small packaging. The video online really helped.
iHealth COVID-19 Self-Test At-Home. –
We were throwing a party for our company and needed these tests last minute. Nitin at Peach Medical assisted us in getting the tests couriered to our party just in the nick of time. Peach really went above and beyond! We will go nowhere else to get our tests!
Iceylis V. –
We were throwing a party for our company and needed these tests last minute. Nitin at Peach Medical assisted us in getting the tests couriered to our party just in the nick of time. Peach really went above and beyond! We will go nowhere else to get our tests!
iHealth COVID-19 Self-Test At-Home. –
Due to union requirements, we have to test all employees weekly. The BinaxNOW was getting expensive for us so we tried IHealth. I would say that BinaxNOW is very easy to use but the IHealth is not too far from it. At half the price, a little training did not hurt our staff. The cell phone application is unique, we may try to implement next year depending on the OSHA guidelines.
Nathan –
Due to union requirements, we have to test all employees weekly. The BinaxNOW was getting expensive for us so we tried IHealth. I would say that BinaxNOW is very easy to use but the IHealth is not too far from it. At half the price, a little training did not hurt our staff. The cell phone application is unique, we may try to implement next year depending on the OSHA guidelines.