



Exp date: January 11th, 2025
$28.00 – $650.00

| Weight | 5.61 oz |
|---|---|
| Dimensions | 8.11 × 3.19 × 2.64 in |
| Weight | 5.61 oz |
| Dimensions | 8.03 x 2.64 x 1.14 in |
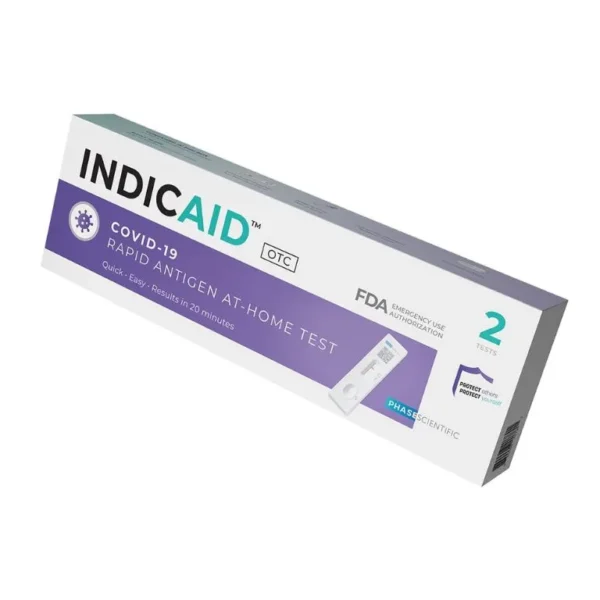
| Brand | INDICAID |
| UPC | 860008407801 |
| Manufacturer | Phase Scientific |
| Package Size | Case (252tests), 4 Kits (8tests) |
| Unit | 1 Case, 4 Boxes |
Items are shipped Monday – Friday from our warehouse. 1-2 Day Processing Time.
Delivery times vary according to your selected delivery address, the availability of your items, and the time of day you place your order.
Orders placed by 1:00 PM Eastern Time will be shipped the same day from our warehouse. Orders placed after this time will be shipped the next business day.
For B2B accounts, please get in touch with us at [email protected] for urgent order requests.
Please feel free to visit our Support Page for FDA Extension Letters, Submit a Ticket, and more.
At Peach Medical Corp we use UPS (most orders ship via UPS but some may ship via USPS) as our logistic and Shipping partners. Currently, our shipping methods are as follows:
Our system updates clients every evening with tracking numbers for their orders. This will be sent via e-mail with an additional copy of your receipt.
Please verify your address when ordering, we are unable to fix an incorrect shipping address on an order once it is in transit. If your order is in transit with an incorrect address, please reach out to your carrier to reroute the package. Peach Medical Corp. is not able to have packages rerouted or contact the carrier for you.
No refunds for packages sent to wrong address. Replacement package may be sent if eligible. Contact customer service for more information.
The Peach Medical COVID-19 Antigen Rapid Test and face masks are personal items. Therefore, they cannot be returned or refunded. Replacement may be shipped if the item is defective. Proof of purchase, photos of defects, lot numbers, and other information are required to authorize a replacement. Please reach out to customer service for detailed requirements before disposing of the defective units. We will not replace your COVID-19 Antigen Rapid Test kits that are expiring soon based on the printed label. Our expiration dates have been shown on each product page. More information can be found at support.peachmedical.com/
We will continue to update the FDA documents for shelf-life extensions for our tests.
If a customer chooses a 3rd party receiver who is not the purchaser, Peach Medical is not responsible for any goods once they leave our warehouse and when tracking is provided. I.e Gifts, International Shipping Services. All Products must be packed in the original, unopened, and unmarked packaging including any accessories, manuals, documentation, and registration that shipped with the Product.
Note: If Peach Medical has not shipped your item within 7 business days, cancellations will be honored. Please email us at [email protected] for return authorization and the shipping address.</P
The INDICAID test has a shelf-life of 15 months from the date of manufacture. The expiration date, which includes a 3-month (90 day) FDA extension, is indicated on the label of the outer box. Learn more.
Yes, our US multi-center clinical study shows that the INDICAID test performs similarly in detecting the COVID-19 Omicron variant compared to the original strain of the virus.
Swab testing is a method used by the biomedical community to collect upper-respiratory specimens for molecular and rapid antigen testing. The CDC endorses this method for its practicality, simplicity, and efficiency in gathering essential data, especially during the COVID-19 pandemic.
COVID-19 tests vary, with molecular tests (PCR) detecting the virus’s genetic material and antigen tests detecting viral proteins. Antigen tests are specific but less sensitive than molecular tests, meaning a positive result is highly accurate, but a negative result does not rule out infection. For symptomatic individuals, serial testing is recommended at least twice over three days (48 hours apart), and for asymptomatic individuals, three times over five days (48 hours apart). Discuss with your healthcare provider whether a molecular test is needed, and refer to the CDC website for the latest guidelines.
Antigen test results should be considered presumptive due to their lower sensitivity compared to molecular tests. Negative results may need confirmation with a molecular test for patient management. Results should be evaluated alongside a patient’s recent exposures, medical history, and clinical signs and symptoms of COVID-19.
Several factors can lead to incorrect test results:
A negative result indicates that viral proteins were not found in your sample, but it is possible to receive a false-negative result. Your healthcare provider will consider the test result along with other medical information to determine the appropriate care. Serial testing is recommended for individuals with negative results: at least twice over three days (48 hours apart) for symptomatic individuals and three times over five days (48 hours apart) for asymptomatic individuals.
The INDICAID test is easy to administer without the need for equipment or training. It has high sensitivity and can detect lower viral load samples compared to other products. The test has been granted Emergency Use Authorization (EUA) by the United States Food and Drug Administration.




PeachMedical Corp
PeachMedical Corp