The 2022-2023 winter season has already seen a nationwide surge in the flu (influenza), COVID-19 variants, and RSV (respiratory syncytial virus) cases. Confirmed cases have begun unseasonably early, a sign that the winter viral season may continue to intensify or even extend longer than usual.
Though multiple factors contribute to this unseasonable pattern, leading experts point to a resounding cause: an immunity gap caused by the pandemic’s public health measures, like masks and social distancing.
“Those [COVID] interventions, while they were great at limiting the spread of COVID-19, they also did a really good job of limiting the spread of other respiratory diseases such as RSV and influenza.
Decreased exposure to endemic viruses created an immunity gap – a group of susceptible individuals who avoided infection and therefore lack pathogen-specific immunity to protect against future infection.”
— Rachel Baker, epidemiologist and assistant professor at Brown University.
Studies have shown that there was an unprecedented drop in RSV cases and hospitalizations in 2020 and 2021, as well as unusually tame flu seasons. Though other factors can exist, experts attribute this reduction in respiratory illnesses to a concerted public effort towards COVID prevention.
And while this was great in 2020 and 2021, both RSV and the flu seem to have returned with a vengeance.
Data from the CDC (Centers for Disease Control and Prevention) show that cases of RSV detected by PCR tests have tripled between August – September 2022, surpassing peak levels from 2021, an extremely unseasonal pattern. Children in particular have been the most susceptible population to RSV, seen in numerous Children’s Hospitals nearing capacity as respiratory illness cases spike nationwide.

The flu, which typically peaks in February, has already pushed hospitalization rates to the highest level for this time of year in more than a decade, and has officially surpassed COVID-19 hospitalizations.
Dubbed the “tripledemic,” public health officials have been warning that the co-circulation and rising hospitalizations of RSV, COVID-19 and the flu could severely strain an already fatigued healthcare system in the United States. Your point-of-care organization may already be experiencing this.
Dr. Larry Kociolek, Medical Director of Infection Prevention and Control, an Attending Physician at Lurie Children’s Hospital of Chicago, recently warned that this unseasonal surge of the flu can hit children under 3 years old the hardest.
“I think [influenza] has the potential to overwhelm our healthcare system,” he cautions, “Influenza essentially went away for the last two and a half years, and so we have a huge population of infants—essentially almost every child in the U.S. who is under 2 and a half to 3 years old—who has not encountered influenza, and the vaccine rate is not particularly good.”
— Dr. Larry Kociolek
In combination with this concern, an unusually high number of RSV infections for this time of year has already resulted in a shortage of tests across America. In a single week of October, over 8,000 RSV tests were reported as positive by the CDFor comparison, that is over double the number of positive results reported for the same week last year.
Those millions will continue to flood their doctor’s offices, hospitals, urgent care facilities, and many other CLIA-approved locations to determine just what it is that is making them feel unwell.
This time last year, multiple tests would be used to determine which virus a patient is suffering from. This year, the medical landscape has evolved thanks to manufacturers,like Lucira Health that have developed advanced combination tests to expedite diagnosis and recovery.
DOWLOAD CLIA-APPROVED PROCUREMENT BEST PRACTICES
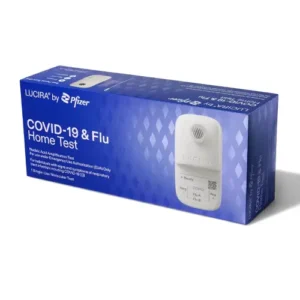
Lucira Health was granted emergency use authorization (EUA) in November 2022 by the US Food and Drug Administration (FDA), making it the first COVID-19 and Flu combination test authorized for use in point-of-care settings.
The Lucira COVID-19 and Flu test uses the same technology and device as their COVID-19 test but has been enhanced to provide independent diagnoses for COVID-19 variants, Flu A, and Flu B. Since this test is a nucleic acid amplification test (NAAT), extremely small concentrations of either virus can be detected in a single swab with quick results, a major differentiator compared to other diagnostic tests on the market. This empowers early detection, which means early treatment.
“COVID-19 and flu viruses can both cause serious illness with similar symptoms, but each has unique prescription treatments that require diagnosis early in the infection to be effective. The inaccuracy of antigen COVID-19 tests makes such tests inadequate to use for early differential diagnosis of flu versus COVID-19.”
— Erik Engelson, President and CEO of Lucira Health.
Available for use in CLIA-waived environments, this easy-to-use, hand-held test delivers results within 30 minutes from a shallow nasal swab. You can expect to see lab-quality positive results in as fast as 11 minutes, allowing for diagnosis during the patient visit.
As a result, “clinicians can now facilitate simultaneous rapid testing at a mass scale to get patients on the path to recovery quickly,” says Engelson.
Prescription treatment of both viruses must begin 2-5 days from symptom onset, making accurate, fast results key to treating COVID variants and flu. Utilizing the Lucira combination test will empower your point-of-care office to treat patients within the window of effective treatment, an advantage that was not always possible previously.
Without the combination test, clinicians must rely on antigen tests that can take too long to detect or PCR tests with delayed results, increasing the risk of viral transmission within your community.

As of December 2022, the Lucira combination test is only available to healthcare providers with CLIA Certification. If your facility is not currently CLIA-approved, you can apply for certification via the Centers for Medicare & Medicaid Services (CMS) Certification Form, CMS-116. This form must be completed and mailed to your state’s regional CLIA office, found on CMS.gov. On average, you can expect to receive your CLIA Certificate within four to six weeks.
If you have any questions about becoming CLIA Certified, please get in touch with us. Our team of consultants can point you in the right direction.
Click Here to Become CLIA-Approved – Learn More Here
Once you have your CLIA Certification, it is vital to partner with a trusted vendor that works with you to not only lock in pricing and a distribution strategy, but one that also provides guaranteed products sourced directly from manufacturers with accurate CPT codes. There have been many instances of counterfeit, faulty or expired tests being distributed to organizations throughout the pandemic, often being shipped from third-party vendors abroad.
Unfortunately, we also typically see medical suppliers increase pricing to meet demand for testing kits and PPE, especially as hospitals become flooded and supplies run dry. This predatory practice has been consistent throughout the pandemic, and likely to appear again, as respiratory illnesses spike over the coming winter months.
We simply do not agree with these practices.
As an authorized, US-based partner of Lucira Health, Peach Medical is working towards bringing the Lucira COVID-19 and Flu test to our point-of-care clients. And because we source directly from manufacturers, we guarantee that all products are authentic, within effective use periods, and will arrive at your facility when you need them.
We work closely with medical experts and manufacturers to stay current on changing conditions to stay ahead of trends in new variants or viral surges. We believe it is our role to advise clients on the changing landscape of virus prevention and provide a plan to execute against if needed. This consultative approach sets Peach apart from other vendors in the industry, as we are an extension of your team and not just a vendor.
Looking to make sure your procurement is CLIA-approved and aligns with seasonal patterns?
Download Strategies for CLIA-Approved Procurement
Disclaimer: Peach Medical strongly recommends discussing any testing plans or protocols with a certified physician or healthcare professional before making any decisions. The information provided by Peach Medical is intended to be general in nature and is not a substitute for professional medical advice, diagnosis, or treatment.





PeachMedical Corp