The need for the early identification of SARS-CoV-2 has let to a quest for reliable tests that meet the standards of polymerase chain reaction (PCR) tests, on the one hand, and are low-cost, easy-to-use, and fast, on the other hand. One such test is the Lucira™ Check It COVID-19 Test kit (“Lucira”) (Lucira Health, Inc., Emeryville, CA, USA), which utilizes real-time loop-mediated isothermal amplification technology, developed for at-home use. This study evaluated the clinical sensitivity and specificity of Lucira in identifying the virus in 190 nasopharyngeal samples collected between January and October 2021. Each sample was also subjected to RT-PCR. All negative RT-PCR results were paralleled by a negative Lucira result. Out of 90 participants who had a positive RT-PCR result, 82 (91.1%) tested positive by Lucira. Among the 72 symptomatic participants, 67 (93%) tested positive by Lucira. All samples with a positive RT-PCR result with a threshold cycle (Ct) > 36, yielded a negative Lucira result. In addition, a significant positive correlation was found between Ct and time-to-positivity with Lucira (R = 0.8612, p < 0.0001). The implementation of such a portable and affordable assay may aid in breaking the COVID-19 transmission chain.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19), originated in China around the end of 2019 and swiftly expanded over the world, infecting about 500 million individuals. Given its high contagiousness, early identification and isolation of affected persons was the primary infection control approach immediately implemented. To halt the pandemic’s spread, it was essential to employ an effective testing technique. In addition, healthcare practitioners were able to conduct clinically effective interventions for individuals at a higher risk of developing major medical consequences when an early diagnosis was made. Clinical manifestations of COVID-19 include respiratory symptoms such as difficulty breathing and pneumonia, as well as non-respiratory symptoms including fever, cough, tiredness, myalgia, headache, and diarrhea . Some patients may appear with no symptoms or mild flu-like symptoms. When considering the adoption of a diagnostic test for SARS-CoV-2, it is necessary to evaluate the impact of symptom variability on test results. The worldwide gold standard for detecting SARS-CoV-2 is the reverse transcriptase-polymerase chain reaction (RT-PCR), a highly sensitive and specific molecular technique that detects viral RNA in respiratory samples. The various RT-PCR tests target one or many genes, such as the nucleocapsid (N), spike (S), and open reading frame 1 beta (ORF1b) genes. Nonetheless, it requires costly laboratory equipment and chemicals, competent laboratory personnel, and considerable time [4]. Furthermore, RT-PCR-based tests demand high-quality RNA samples.
Due to the severity of the epidemic, limited molecular test infrastructure capacities, and reagent shortages in many countries, the development of rapid and simple-to-use SARS-CoV-2 detection tests was a necessity. One such assay is the fast antigen test, which is based on the identification of specific viral proteins or antiviral antibodies. These tests are quick, inexpensive, appropriate as point-of-care (POC) assays, and do not require highly trained laboratory staff. However, most have lower sensitivity and specificity than RT-PCR, particularly in samples from asymptomatic patients or those with low viral loads. Reverse transcriptase loop-mediated isothermal amplification is a second form of quick SARS-CoV-2 detection assay (RT-LAMP). Although antigen testing are less expensive, RT-LAMP is more precise [7]. Utilizing a specific DNA polymerase to conduct a PCR reaction at a consistent temperature is novel to this approach. This eliminates the need for a thermal cycler and permits rapid and efficient amplification. The LAMP approach is particularly specific since it can recognize as many as six different locations on the viral RNA [8]. The reaction consists of two phases: the initial structure-forming phase and the cycling amplification phase. The initial structure-generating process necessitates the utilization of four specifically built, target-specific primers (inner primers: FIP and BIP and outer primers: F3, and B3). The Bst DNA polymerase commences DNA synthesis from FIP, after which the F3 primer binds to the target DNA and the newly synthesized strand displaces the target DNA, which is subsequently released. The released strand’s 30th end then forms a loop shape.
Next, a new strand is synthesized using the BIP and B3 primers in the same fashion as the FIP and F3 primers, and a second loop structure is produced to create a dumbbell-like structure. The subsequent amplification stage begins with the conversion of the dumbbell-like structure to stem– loop DNA by self-priming DNA synthesis and continues with strand displacement DNA synthesis to generate a second dumbbell-like structure. Further elongation and recycling of strep reactions result in the desired elongated products.
In less than 30 minutes, the new LuciraTM Check It COVID-19 Test kit (“Lucira”) (Lucira Health, Inc., California, United States) can detect SARS-CoV-2 from a few copies of RNA. Based on clinical testing conducted by the manufacturer in comparison to laboratory PCR procedures, the accuracy of the kit was 98% [10]. The kit has Emergency Use Authorization (EUA) from the FDA for home use by individuals aged 14 and older or for use at the point of care (POC) by healthcare providers for all ages [11]. Among the three commercially available molecular tests with an EUA authorization, the Lucira kit and the Detect Start Kit cost USD 75, while the Cue is significantly more expensive; despite the fact that the tests are sold in 3 or 10 test packs, which may reduce test costs, only the Cue Reader costs USD 249. While these POC tests are often less expensive than a PCR test, they are 3–10 times more expensive than an antigen test. Note, however, that antigen tests are less sensitive than molecular-based techniques.
In the present investigation, we investigated the clinical sensitivity and specificity of Lucira by comparing its results to those of RT-PCR using symptomatic and asymptomatic patient samples. According to our knowledge, this is the first evaluation of the performance of the Lucira.
The RT-PCR was designated as the standard method for calculating the sensitivity and specificity of clinical tests. Consequently, specimens that tested positive or negative via RT-PCR were designated as “True Positive” or “True Negative,” respectively. The Spearman correlation coefficient was calculated to determine the relationship between the RT-PCR Ct value and the Lucira time-to-positive.
Patients with RT-PCR+/Lucira results and patients with RT-PCR+/Lucira+ results had their distribution of Ct values (RT-PCR) compared using the nonparametric Mann–Whitney test.
A p value of 5% was regarded as statistically significant for all two-tailed tests. GraphPad Prism version 5.03 was used to analyze the data.
The study included 190 participants, of whom 100 (52.6%) tested negative for SARSCoV-2 RNA by RT-PCR and the rest were positive.
Eighty percent (72) of the positive group exhibited some of the clinical signs of COVID-19 infection, including gastrointestinal symptoms, weakness, dry cough, and/or dyspnea.
All subjects who tested negative with the RT-PCR also tested negative with the Lucira. Among the 90 subjects whose RT-PCR results were positive, 82 (91.1 %) had a positive Lucira result. 67 (93%) of the 72 symptomatic RT-PCR-positive subjects tested positive with Lucira. Table 1 presents a summary of the outcomes. Overall, the clinical sensitivity of Lucira was 91.1% (CI: 83.23–96.08) and its clinical specificity was 100%. (CI: 96.38–100).
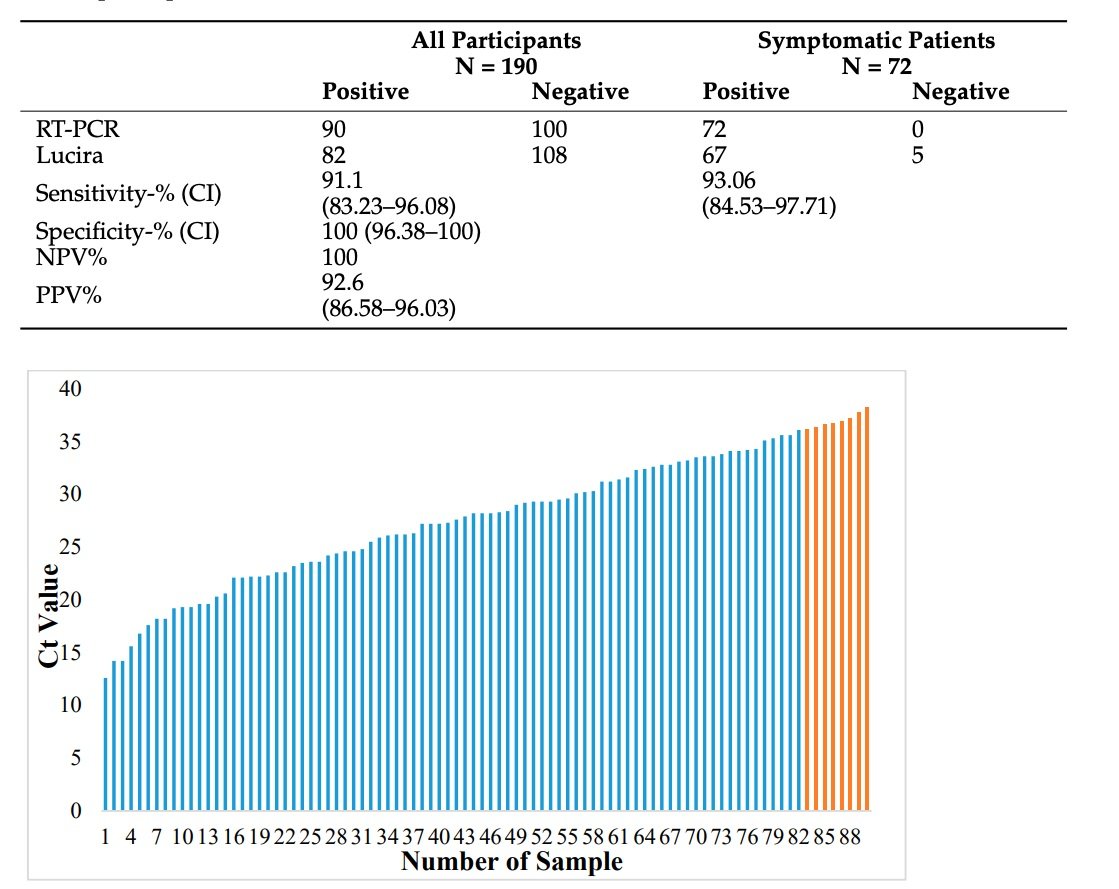
Among symptomatic patients, the clinical sensitivity of Lucira was 93.06%. (CI: 84.53–97.71). All patient samples with a Ct value greater than or equal to 36 revealed negative Lucira results. These eight samples showed a mean Ct value of 37, which was considerably higher than the mean Ct value of samples from patients with RT-PCR+/Lucira+ findings (26.8) (p 0.001).
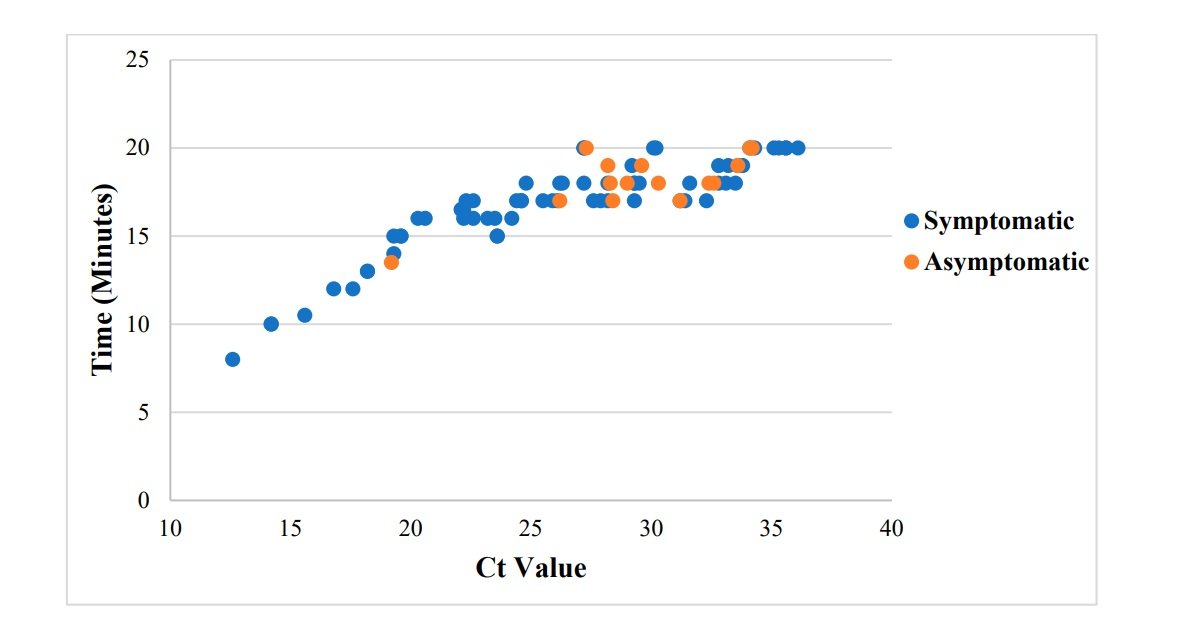
Analysis of time-to-positivity (in the Lucira) of samples from symptomatic and asymptomatic patients revealed that the average time-to-positivity for samples from symptomatic patients was 16.8 minutes, whereas the average time-to-positivity for samples from asymptomatic patients was 18.1 minutes. Less time was required for Lucira to exhibit a positive result the lower the Ct value (in RT-PCR) (p 0.0001, R = 0.8612). (0.7898–0.9096)
The primary objective of this study was to evaluate the clinical sensitivity and specificity of the LAMP-based LuciraTM Check It COVID-19 Test Kit. The results of the kit were compared to those of the most sensitive FDA-approved RT-PCR tests for SARSCoV-2 detection.
The study included both symptomatic and asymptomatic individuals, as well as participants negative for COVID-19.The clinical sensitivity of Lucira was high (91.1%) and Slightly higher when only symptomatic patients are included (93.06%). Previous investigations have indicated a wide range of sensitivities for the LAMP assay (from 67% to 100%); however, some of these research used RT-LAMP on extracted RNA rather than the original sample, or employed a relatively small sample size [18]. The vast majority of LAMP tests exhibited high specificity values (97–100%)ddd. As with the majority of other FDA-approved molecular POC SARS-CoV-2 assays, the Lucira kit demonstrated excellent clinical performance. Compared with other LAMP-based POC test, the “Detect COVID-19 Test” (Detect Inc., Panama City, FL, USA) demonstrated slightly higher positive and negative percent agreement compared to the EUA comparator assay and a higher analytical sensitivity. The Talis One COVID-19 test, which is similarly LAMP-based, has the same characteristics. Excellent clinical performance; however, it has not been approved for home use.
All false negative Lucira results were obtained from patients whose RT-PCR results had a Ct value greater than 36, which implies a low viral load. These results indicate that Lucira is more accurate in symptomatic patients than in asymptomatic individuals and in patients with a moderate to high viral load. Consequently, Lucira may be helpful for the detection of infectious individuals who should be segregated. Our findings are corroborated by prior research.
There was a significant correlation between Ct values of RT-PCR and time-to-positivity of Lucira results (p 0.0001); in samples of patients whose PCR result had low Ct values, indicating a high viral load, the positive result in Lucira was displayed in a shorter period of time compared to samples with a Ct value > 36. This study further supports the idea that Lucira performs better in patients with a moderate-to-high viral load and a greater likelihood of transmitting the virus. In a previous study, Kidd and colleagues discovered a smaller association between Ct values and time-to-positivity (R = 0.431); however, Kidd et al. evaluated all samples, including RT-LAMP-negative ones, whereas we calculated the correlation based on positive samples only.
Despite its great accuracy, which has made RT-PCR the gold standard for detecting SARS-CoV-2, RT-PCR is time-consuming and costly. Even the most recent PCR-based technologies that do not necessitate RNA extraction still require expensive, non-portable, specialized equipment. Due to its excellent precision and user-friendliness, the Lucira kit can be utilized in institutions requiring a speedy COVID-19 result, such as airports, emergency rooms, and intensive care units. The kit has several advantages; first, it was developed for home usage, enabling self-testing without the need for specialist equipment or highly qualified individuals to interpret the data. Moreover, findings are displayed within thirty minutes. Therefore, it can be utilized as a point-of-care test.
The Lucira kit is advantageous owing to its high clinical sensitivity (91.1%) and specificity (100%) compared to RT-PCR. Among the symptomatic patients, Lucira’s clinical sensitivity was 93.06. It gives precise test results in a short amount of time. We believe that the use of this portable and cost-effective kit could aid in early diagnosis and contribute to the avoidance of additional SARS-CoV-2 virus spread.
Disclaimer: Peach Medical strongly recommends discussing any testing plans or protocols with a certified physician or healthcare professional before making any decisions. The information provided by Peach Medical is intended to be general in nature and is not a substitute for professional medical advice, diagnosis, or treatment.





PeachMedical Corp