As we enter the US holiday season, medical experts nationwide warn of a surge across three viruses: the Flu (influenza), COVID-19 variants, and RSV (respiratory syncytial virus). Dubbed the “tripledemic,” leading experts are particularly concerned about how the Flu and RSV will impact our most vulnerable – children.
Dr. Larry Kociolek, Medical Director of Infection Prevention and Control, an Attending Physician at Lurie Children’s Hospital of Chicago, recently warned Fortune Magazine about the upcoming Flu season, citing a concern around lack of exposure amongst children under 3 years old:
“I think [influenza] has the potential to overwhelm our health care system,” he cautions. “Unlike RSV, enterovirus—which is a group of infections that cause mild to serious illnesses—and COVID, we haven’t had an influenza season since early 2020. Influenza essentially went away for the last two and a half years, and so we have a huge population of infants—essentially almost every child in the U.S. who is under 2 and a half to 3 years old—who has not encountered influenza, and the vaccine rate is not particularly good.”
— Dr. Larry Kociolek
In combination with this concern, an unusually high number of RSV infections for this time of year, has already resulted in a shortage of tests across America. In a single week of October, over 8,000 RSV tests were reported as positive by the CDC (Centers for Disease Control and Prevention). For comparison, that is over double the number of positive results reported for the same week last year.
All three viruses, COVID-19, Flu, and RSV, are considered to be highly contagious respiratory illnesses with similar symptoms. This means you can test negative for COVID and the Flu, but be positive for RSV.
According to pediatrician Dr. Ira Wardon of Providence Cedars-Sinai Tarzana Medical Center, “we are already seeing patients testing positive for more than one virus.” Given the potential to contract all three in tandem, it is imperative that organizations, especially theater companies and those in the performing arts, have access to the appropriate testing for all three viruses.
Theaters, ballet companies, children’s theaters, and performing arts centers often cater to families with children during the holidays, who run the risk of contracting viruses when attending shows.

For good reason. The pandemic has been marked with hundreds of canceled shows and tours as a result of outbreaks as well as vendor failures.
Just recently, the Kia Forum announced a rescheduling of Harry Styles’ November 4, 2022 show, citing band illness as the primary reason. Cancellations and delays are not limited to musical performances either. According to Playbill, the Syracuse Stage has been forced to cancel the remainder of its season opener’s run, the world premiere of musical How to Dance in Ohio, due to “a number of COVID cases within the company.” The production’s opening date was September 23, 2022.
Whilst outbreaks can be a cause of failed protocols, they also result when vendors responsible for testing kits and personal protective equipment (PPE) fail to deliver quality products on time. Often, third party vendors will deliver faulty, expired testing kits to organizations – and they do not replace tests for clients, resulting in a poor investment for performing arts organizations.
This is further exacerbated when show tours are taken into consideration – multi-site distribution is a major weakness of most third party resellers since they do not warehouse their products in-house. This results in significant shipment delays, severely impacting a theater’s preventative testing policies and union compliance.
Combining best practices learned from our performing arts clients, union policies, and expert advice from leading medical practitioners, we’ve put together five key strategies that will help any theater respond and survive the tripledemic.
Most theaters are familiar with preventative screening and surveillance testing, but many implement blanket requirements across all staff and locations. The reality is that each team, from talent to backstage crew to production, are at varying degrees of risk based on their roles. For example, talent does not typically wear masks during rehearsals and performances, and often, must be physically close to one another to deliver a scene well. According to the World Health Organization (WHO), Covid transmission primarily occurs “between people who are in close contact with each other, typically within 1 meter,” which translates to about 3 feet of space. Our health consultants recommend that employees with close contact, like talent teams, test at an increased frequency “with a high quality portable PCR equivalent test along with antigen tests depending on the use case”.
CLICK HERE TO CREATE A UNION-COMPLIANT TESTING CADENCE
Screening for COVID, RSV, and the Flu requires broad-scale testing of your theater staff. This helps identify individuals who are infected before they are in contact with others, and has proven to decrease the likelihood of large outbreaks when coupled with quarantine requirements. Screening measures are typically in place prior to the commencement of a show – before rehearsals even begin.
Surveillance testing differs in that it seeks to monitor general infection rates over time. This approach often requires recurring testing to determine if the rate of infection is increasing or decreasing. This method empowers theaters to determine if an outbreak has started and should result in an adjustment to health measures to curb it. This data should be closely monitored by COVID Compliance Officers – as the overall rate of infection increases, theaters must take action to curtail an outbreak.
According to Helix Research, the success of screening and surveillance testing measures is completely contingent on the availability of tests with a rapid turnaround time. The longer turnaround is, the more likely infected individuals spread the virus to others. As a general rule, Peach Medical recommends tests with ideally a 15 min turn around and definitely no more than a 24 hour time to result.
Depending on the size of your theater, testing measures will differ. The square footage of your space dictates physical proximity, which will impact the likelihood of transmission during an exposure. When determining your preventative testing cadence, it is imperative that your vendor works with you to project the quantity and frequency of testing kits for your theater based on theater size, number of employees, types of teams, and frequency of rehearsals and performances. In addition, your testing cadence should be flexible enough to respond to infection rate changes. Continue reading to determine how you can read national and internal infection statistics to adjust testing protocols.
2. Leverage Infection Rate Statistics to Drive Testing Measures
The CDC aggregates and updates verified cases of COVID across geographic areas in the United States on a daily basis to visualize risk levels in its hotspots map. More recently, the CDC has broadened the scope to monitor visits for respiratory illness symptoms, which includes potential cases of RSV, COVID variants, and the Flu. This data, in particular the Outpatient Respiratory Illness Activity Map, is extremely important to review for COVID Compliance and Health Safety Managers, as it can help you determine if a locale your theater or show is performing in is at risk. These maps also empower you to determine when and where you will need testing kits sent, especially in the case of traveling shows and multiple theater locations.
As a city or county begins to trend towards more reported cases and respiratory illness-related doctor visits, your theater can take action by proactively procuring testing kits for the region regardless of your existing testing cadence. In other words, if San Francisco is experiencing a surge in positive cases, theaters in and around the Bay area will need to increase access to testing kits while in tandem increasing the frequency of their testing cadence.
In short, more cases mean more testing. Seems simple, right? This common sense approach to testing is made more complicated when we factor in that most third party resellers will increase price points during a surge and will have less testing kits as a result of the increased demand for the impacted region. This often results in theaters not being able to procure tests when they need it most.
The best way to protect your theater from this exploitative approach is to negotiate long term contracts with vendors to ensure your price point is honored during times of surge. For example, Peach Medical typically will hold fresh supply for our clients and distribute it as needed based on anticipated usage and surge demand, all at the price point set on day 1.
As with national data, your internal infection rate statistics will also guide adjustments to your testing cadence. As surveillance testing begins to show an upward trend in positive cases, your theater’s health safety measures must respond accordingly. This means increased testing for all staff and quarantine requirements to curtail a major outbreak
3. Choose Chain of Custody Visibility for Effective Tests
The term “chain of custody” is often used to describe the supply chain process that tracks the movement and lifecycle of products. Due to the large number of third party resellers purchasing test kits from overseas, this kind of visibility could mean the difference between faulty tests and effective, guaranteed tests.
We often hear of third parties relabeling test kits to mask counterfeit product, an expiration date, or temperature requirements, a practice that can cause an unassuming theater to fall out of compliance with unions. When working with a vendor, it is imperative to determine where products are sourced from, how they are stored, what temperature controls are in place, and what happens to expired tests.
In an ideal world, your vendor should source directly from manufacturers, like Abbott Laboratories, store all products in temperature controlled trucks and warehouses, and stay up-to-date with FDA test extensions. In addition, we recommend negotiating terms that require your vendor to replace any faulty tests within 24 hours, a practice we honor for all Peach clients.
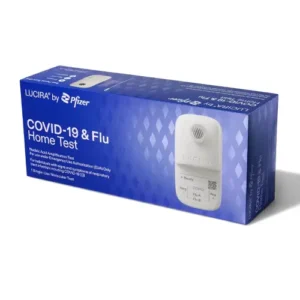
When your vendor is certified to source and sell directly from manufacturers, you gain an advantage when it comes to new tests coming to market. For example, Lucira Health, one of our partners, was recently approved to distribute an at-home Flu and COVID-19 combination test in Canada. As of November 22, 2022, Lucira is now able to sell this combination test in the United States. Due to our existing relationship and the operating practices in place, Peach Medical is positioned to distribute the Lucira COVID-19 & Flu Test to CLIA-approved organizations once the manufacturer’s distribution strategy has been defined.
In addition to where test kits come from, your theater is responsible for ensuring testing is LAMP-approved in accordance with union requirements. This is a kind of diagnostic test that is molecular based, which is more accurate than antigen based testing and equally accurate to lab based PCR testing. While we recommend LAMP testing to our theater clients, rapid antigen testing is also important to double check false positives or faulty results.
4. Adhere to the Strictest Union Requirements
When multiple unions are requiring various protocols, which do you follow? It’s a question that stumps many of the theater Health Safety teams we work with. The best practice, as implemented across numerous theaters we support, is to adhere to the strictest requirements on the table.
Our clients will often share their list of unions with us, so that we may do the heavy lifting of reviewing requirements and providing a recommendation that will be approved by their theater unions. This review is supervised by our in-house board-certified and Mayo Clinic trained Chief Medical Officer, Dr. Kirk O’Donnell, who is the Medical Director for USA Boxing’s Olympic Team and who designed testing protocols for the US Olympic and Paralympic Committee including the Tokyo summer games.
According to Dr. Kirk, “It is critical to have a well designed protocol that takes into consideration cost, location, test type, patient population characteristics, and the organization’s goals as investing large amounts of time and consideration in an intelligently designed protocol and adjusting carefully and in real time as needed can pay dividends down the road financially as well as in terms of organizational productivity, risk/liability mitigation and, most importantly, in terms of the health and safety of the groups being tested and their loved ones.”
Each union will likely have their own mitigation plan as Community Risk Levels fluctuate. The changes to controls can and should be aligned to your procurement strategy for test kits and PPE. If your theater moves from medium to high risk, the higher-level controls your unions outline typically result in an increase in testing frequency, which means your theater must be able to swiftly procure the necessary tests and equipment to stay compliant. This requires your vendor to have quality products at a moment’s notice. To best support our clients when they need it most, Peach Medical stores test kits and PPE in our own warehouses with guaranteed same-day shipping (if ordered prior to 2PM ET).
“Whatever it is, the way you tell your story online can make aWe have clients that may need thousands of test kits across 5 different theaters, and when they need it, they need it now. We’ve optimized our operations to be as nimble as possible while guaranteeing quality – all products are stored in a clean, temperature-controlled environment with maximum, daily oversight”
— states Peach Medical’s Chief Procurement Officer, Adam Malik
Most, if not all, unions will require a series of protocols that Safety Managers must follow when it comes to pandemic operations. The most standard types of requirements we see pertain to:
For a full playbook on how to navigate union requirements
Download “What Every Theater Needs to Stay Union Compliant.”
5. Source from a Consultative Vendor
If there is one thing the pandemic has shown us, it’s that information is rapidly evolving and organizations are expected to evolve with it. Safety Managers must work to stay ahead of the curve and anticipate what may come next to ensure the health and safety of their theater productions. But with the FDA providing new extensions on test kits, surges in a medley of respiratory viral illnesses, and changing union pandemic requirements, it can be a lot to keep up with.
Here at Peach Medical, we work closely with medical experts and manufacturers to stay current on changing conditions to stay ahead of trends in new variants or viral surges. We believe it is our role to advise clients on the changing landscape of virus prevention and provide a plan to execute against if needed. This consultative approach sets Peach apart from other vendors in the industry, as we are an extension of your team and not just a test kit vendor.
By implementing a proactive approach to testing, analyzing Community Risk Levels regularly, and adhering to your strictest union’s pandemic protocols, your theater can be well-positioned to navigate the “tripledemic” with minimal outbreaks. The key is to stay close to the data and work with a vendor that will help set you up for success.
In need of a union-compliance playbook to ensure your current pandemic operations are successful?
Download our Complete Theater Playbook for Health Safety here
Disclaimer: Peach Medical strongly recommends discussing any testing plans or protocols with a certified physician or healthcare professional before making any decisions. The information provided by Peach Medical is intended to be general in nature and is not a substitute for professional medical advice, diagnosis, or treatment.





PeachMedical Corp